Abstract
Background: Intensive chemotherapy regimens (IC; e.g., 7+3 or CPX-351) broadly target leukemic blast populations in acute myeloid leukemia (AML) by interfering with DNA replication, whereas hypomethylating agent/venetoclax therapy (HMA/ven) takes advantage of metabolic vulnerabilities in leukemic stem cells via inhibition of anti-apoptotic pathways. IC regimens are often targeted to 'fit', younger patients, while HMA/ven is typically used in patients who are older or who have significant comorbidities. Both therapeutic paradigms can induce morphologic remission, with resultant measurable residual disease (MRD) status remaining a prognostically significant factor. Here, we aim to determine whether post-remission outcomes in AML patients depend mainly on MRD status or are further modified by chosen induction (IC vs HMA/ven).
Methods: Retrospective chart review was performed on adult (age 18 years and older) patients newly diagnosed with AML and treated at Johns Hopkins Hospital, Baltimore, MD between September 2014 and June 2021. Patients were excluded if they had promyelocytic or mixed phenotype leukemia. Complete remission (CR) was defined by <5% medullary blasts with no extramedullary disease and count recovery. Primary covariates of interest included age in years, performance status (PS; ECOG 0-1 for good vs 2-4 for poor), European LeukemiaNet (ELN) 2022 risk group, number of HMA/ven cycles to best response, hematopoietic stem cell transplantation (HCT) status, chemotherapy type (IC vs HMA/ven), and MRD status by multiparametric flow cytometry (MFC). Groups were compared using Wilcoxon rank sum, Kruskal-Wallis, and Fisher's exact tests. Laplace regression models were used to estimate subgroup median overall survivals (OS) in order to compare them using the Wald test. Associations between OS and the above covariates were examined in Cox models; one multivariable model included an interaction term for therapy type and MRD status. Analyses were performed using Stata version 17.
Results: Of the 388 patients initially reviewed, 214 (55%) achieved a CR or CR with incomplete count recovery and were included in further analysis. Most (103 of 159) IC patients received 7+3; 20 received CPX-351 and 36 received a high-dose cytarabine-based regimen. Fifty-five patients received HMA/ven. The HMA/ven patients were significantly older than those receiving IC regardless of MRD status (median age 72 vs 58 years, respectively, P<0.001). MRD negative (MRD-) remission was achieved in 134 (84.3%) patients after IC and 44 (80.0%) patients after HMA/ven. Patients who achieved MRD- CRs after IC were more likely to undergo HCT than those with identical MRD status after HMA/ven (77.6% vs 40.9%, P<0.001) and were younger (median age 60 vs 73 years, P<0.001).
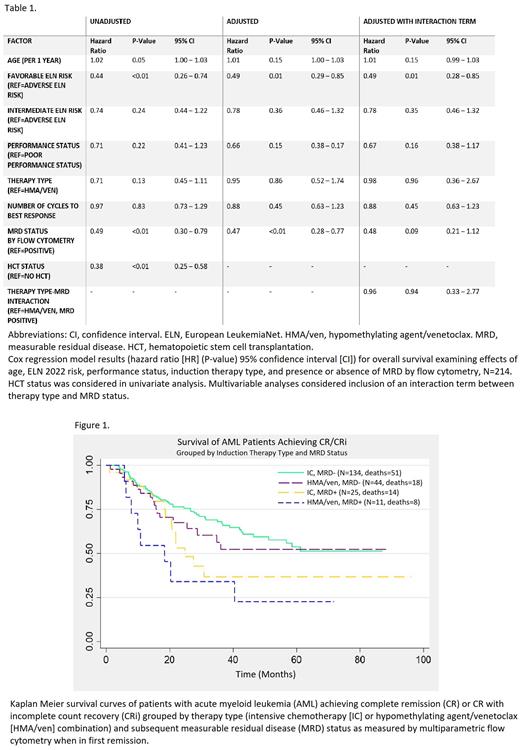
Univariate analysis showed significantly decreased hazard of death in patients with favorable ELN 2022 risk score, MRD- status in first CR, and HCT (Table 1). Kaplan-Meier curves showed patients with MRD- remissions had superior survival to those with MRD positive (MRD+) remissions (median OS not reached vs. 21.9 months, P<0.05), whereas there was no statistically significant difference in median OS between therapies (36.1 months HMA/ven vs. 61.2 months IC). Patients in the IC, MRD- group and HMA/ven, MRD- group had similar outcomes (median OS not reached for both); both groups had superior survival to MRD+ groups (P<0.05; Figure 1).
Multivariable analysis showed decreased hazards of death for ELN favorable risk patients (hazard ratio [HR] 0.49, 95% confidence interval [CI] 0.28-0.85) and those MRD- in first CR (HR 0.47, 95% CI 0.28-0.77) after adjusting for age, PS, therapy type, and cycles. In a subsequent model, an interaction term between therapy type and MRD status was not significant (HR=0.96, suggesting no difference in impact of MRD between therapy types).
Conclusions: In this retrospective study evaluating outcomes in patients with AML, chemotherapy type does not appear to differentially impact survival by MRD status in those achieving remission. MRD status as measured by MFC significantly impacts survival when adjusting for relevant baseline covariates. These results suggest that obtaining deep, MRD- remissions matters regardless of whether these are achieved with HMA/venetoclax or intensive therapy. Ultimately, randomized controlled trials are needed to better determine the value of MRD in AML after different induction strategies.
Disclosures
DeZern:Syntrix Pharmaceuticals: Research Funding; CTI BioPharma: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; GERON: Other: DSMB.
Author notes
∗Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal